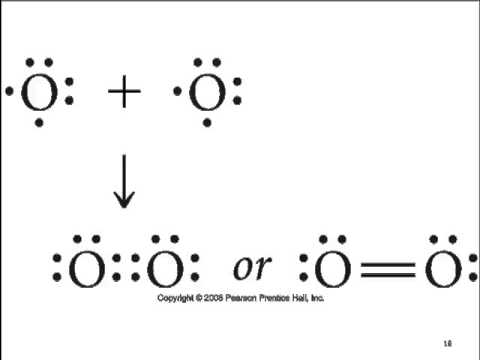CSET Study Guide Chemistry Subset III (121) > 1.3b Molecular Structure and Chemical Bonds - Lewis DotDraw Lewis Dot Structures for Compounds and Ions (1.3b) - Lewis Structures are visual representations of the bonds between atoms and illustrate the lone pairs of electrons in molecules. They can also be called Lewis dot diagrams and are used as a simple way to show the configuration of atoms within a molecule.
- Lewis Theory
- Between 1916 and 1919, Gilbert Newton Lewis, Walther Kossel, and Irving Langmuir came up with a theory to explain chemical bonding.
- This theory would be later called Lewis Theory and it is based on the following principles:
- Valence electrons, or the electrons in the outermost electron shell, have an essential role in chemical bonding.
- Ionic bonds are formed between atoms when electrons are transferred from one atom to another. Ionic bond is a bond between nonmetals and metals
- Covalent bonds are formed between atoms when pairs of electrons are shared between atoms. A covalent bond is between two nonmetals.
- Electrons are transferred/shared so that each atom may reach a more stable electron configuration i.e. the noble gas configuration which contains 8 valence electrons. This is called octet rule.
- Lewis Symbols and Lewis Structures
- A Lewis Symbol for an element is composed of a chemical symbol surrounded by dots that are used to represent valence electrons.
- An example of a Lewis symbol is shown below with the element Carbon, which has the electron configuration of 1s22s22p2:
- This Lewis symbol shows that carbon has four valence electrons in its outer orbital and these four electrons play a major role in bonding of carbon molecules.
- Lewis symbols differ slightly for ions
- When forming a Lewis symbol for an ion, the chemical symbol is surrounded by dots that are used to represent valence electrons, and the whole structure is placed in square brackets with superscript representing the charge of the ion.
- An example of a Lewis symbol for the cation and anion of Carbon is shown below:
- Constructing Lewis Structures
- To construct Lewis Structures one can generally abide by the following steps:
- Find how many valence electrons (N) are in the molecule that needs to be shown on the Lewis Structure by using the periodic table. Find the charge, add an electron for every negative charge and remove an electron for every positive charge.
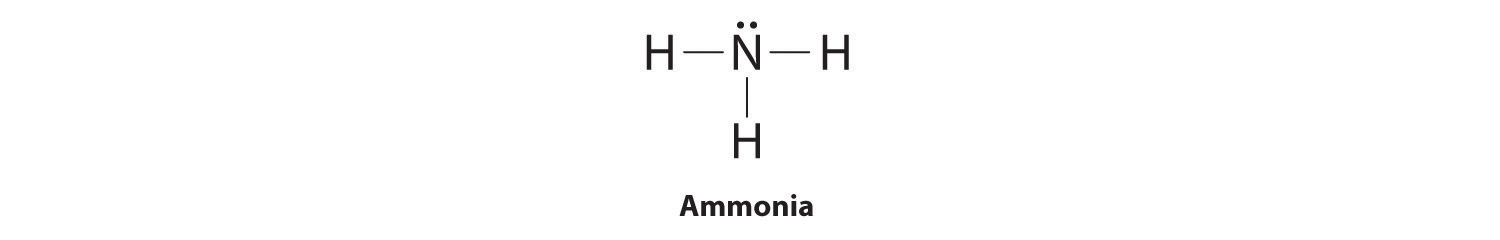
- Draw out the single bonds and initial framework, called the skeleton, of the molecule.
- Complete the octets around the non-central atoms i.e. the terminal atoms by using the lone-pairs of electrons.
- Compare the number of electrons currently depicted to the number needed (N) in the central atom and add electrons to it if less the number is less than N.
- If there are extra lone-pair electrons and the octet rule is not filled for the central atom, use the extra electrons to form double or triple bonds around the central atom.
- Check the formal charge of each atom (Formal Charge explained below).
- When constructing the structures keep in mind the following:
- The dots surrounding the chemical symbol are the valence electrons, and each dash represents one covalent bond (consisting of two valence electrons)
- Hydrogen is always terminal in the structure
- The atom with the lowest ionization energy is typically the central atom in the structure
- The octet rule means there are 8 valence electrons around the atoms, but for hydrogen the maximum is 2 electrons
- Another way to look at the method for forming a Lewis Dot Structure
- add the number of valence shell electrons
- if anion, add the charge to valence shell electron number
- if cation, subtract the charge from the valence shell electron number
- write the symbol for each atom showing how they connect to each other
- draw a single bond between each pair on connected atoms
- place remaining electrons around the atoms as unshared pairs (lone pairs)
- if every atom has octet (except H, which has 2) then Lewis Structure is complete
- if too few, draw multiple bonds until octet has formed
- if too many, then the octet rule is broken for this compound
- Lewis Structures can differ based on whether the electrons are shared through ionic or covalent bonds.
- An example of covalent bonding can be seen below with the reaction of Hydrogen and Fluorine:
- Hydrogen has one valence electron and Fluorine has seven valence electrons; together the elements form the noble gas configuration.
- The Hydrogen atom shares its electron with Fluorine atom so that the Hydrogen atom has 2 electrons and the Fluorine atom has 8 electrons.
- Therefore both atoms have their outermost shells completely filled.
- The charge on each atom in a molecule is called the formal charge. The formal charge can be calculated if the electrons in the bonds of the molecule are equally shared between atoms. This is not the same thing as the net charge of the ion.
- In calculating formal charge, the following steps can be extremely helpful:
- Determine the number of valence electrons that should be present for each atom in the structure.
- Count the electrons around each atom in the structure (each lone pair = 2 electrons, each single bond =1 electron, each double bond = 2 electrons, each triple bond = 3 electrons).
- Subtract the number of valence electrons that should be present (from step 1) from the electrons counted in step 2 for each atom. This is the formal charge for each atom.
- Check that the formal charges add up to equal the overall charge of the molecule.
Formal charge = (number of valence electrons) - (number of non-bonding electrons + 1/2 number of bonding electrons) - In Lewis structures, the most favorable structure has the smallest formal charge for the atoms, and negative formal charges tend to come from more electronegative atoms.
- An example of determining formal charge can be seen below with the nitrate ion, NO3-:
- The double bonded O atom has 6 electrons: 4 non-bonding and 2 bonding (one electron for each bond). Since O should have 6 electrons, the formal charge is 0.
- The two singly bonded O atoms each have 7 electrons: 6 non-bonding and 1 bonding electron. Since O should have 6 electrons, and there is one extra electron, those O atoms each have formal charges of -1.
- The N atom has 4 electrons: 4 bonding and 0 non-bonding electrons. Since N should have 5 electrons and there are only 4 electrons for this N, the N atom has a formal charge of +1.
- The charges add up to the overall charge of the ion. 0 + (-1) + (-1) + 1 = -1. Thus, these charges are correct, as the overall charge of nitrate is -1.
- Resonance
- There are times when more than one acceptable Lewis structure can be drawn for a molecule and no single structure can represent the molecule entirely.
- When this occurs the molecule/ion is said to have resonance. The combination of the various plausible Lewis structures is called a resonance hybrid.
- Some rules for drawing resonance structures are as follows:
1.The same number of electronsmust be present for all resonance structures. 2.The octet rule must be obeyed. 3.Nuclei (or the chemical symbolin the structure's representation) cannot be rearranged; only the valenceelectrons differ for resonance structures. |
|
Learning Objectives
‹ Lewis Dot Structures up Resonance Structures › These tutorials are sponsored by PhySy, the maker of PhySyCalc on iPhone, iPad, or Mac OS, and RMN on Mac OS. PhySyCalc is the only calculator app that let's you use units directly in calculations. Lewis dot diagrams for elements are a handy way of picturing valence electrons, and especially, what electrons are available to be shared in covalent bonds. The valence electrons are written as dots surrounding the symbol for the element: one dot is place on each side first, and when all four positions are filled, the remaining dots are paired with one of the first set of dots, with a maximum.
By the end of this section, you will be able to:
- Write Lewis symbols for neutral atoms and ions
Lewis Symbols of Monoatomic Elements
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful.
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. (It does not matter what order the positions are used.)
For example, the Lewis electron dot diagram for calcium is simply
Figure 1 shows the Lewis symbols for the elements of the third period of the periodic table.
Figure 1. Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table.
Lewis symbols can also be used to illustrate the formation of cations from atoms, as shown here for sodium and calcium:Likewise, they can be used to show the formation of anions from atoms, as shown below for chlorine and sulfur:Figure 2 demonstrates the use of Lewis symbols to show the transfer of electrons during the formation of ionic compounds.
Figure 2. Cations are formed when atoms lose electrons, represented by fewer Lewis dots, whereas anions are formed by atoms gaining electrons. The total number of electrons does not change.
Example 1: Writing Lewis DoT SYmbols of Elements
What is the Lewis electron dot diagram for each element?
- aluminum
- selenium
Show AnswerThe valence electron configuration for aluminum is 3s23p1. So it would have three dots around the symbol for aluminum, two of them paired to represent the 3s electrons (or three single dots around the atom):
The valence electron configuration for selenium is 4s24p4. In the highest-numbered shell, the n = 4 shell, there are six electrons. Its electron dot diagram is as follows:
Check Your Learning
What is the Lewis electron dot diagram for each element?
- phosphorus
- argon
Lewis Dot Diagram Calc
Show Answer
Example 2: Writing Lewis DoT SYmbols of Ions

What is the Lewis electron dot diagram for each ion?
- Ca2+
- O2−
Show AnswerHaving lost its two original valence electrons, the Lewis electron dot diagram is just Ca2+.
Ca2+
The O2− ion has gained two electrons in its valence shell, so its Lewis electron dot diagram is as follows:
Check Your Learning
The valence electron configuration of thallium, whose symbol is Tl, is 6s25d106p1. What is the Lewis electron dot diagram for the Tl+ ion?
Show Answer
Key Takeaways
- Lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol.
- Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions) dots than the corresponding atom.
Exercises
1. Explain why the first two dots in a Lewis electron dot diagram are drawn on the same side of the atomic symbol.
2. Is it necessary for the first dot around an atomic symbol to go on a particular side of the atomic symbol?
3. What column of the periodic table has Lewis electron dot diagrams with two electrons?
4. What column of the periodic table has Lewis electron dot diagrams that have six electrons in them?
5. Draw the Lewis electron dot diagram for each element.
a) strontium
b) silicon
6. Draw the Lewis electron dot diagram for each element.
a) krypton
b) sulfur
7. Draw the Lewis electron dot diagram for each element.
a) titanium
b) phosphorus
8. Draw the Lewis electron dot diagram for each element.
a) bromine
b) gallium
9. Draw the Lewis electron dot diagram for each ion.
a) Mg2+
b) S2−
10. Draw the Lewis electron dot diagram for each ion.
a) In+
b) Br−
11. Draw the Lewis electron dot diagram for each ion.
a) Fe2+
b) N3−
12. Draw the Lewis electron dot diagram for each ion.
a) H+
b) H−
Show Select Answer1. The first two electrons in a valence shell are s electrons, which are paired.
3. The second column of the periodic table
5.
a)
b)
Lewis Structure Finder
7.
a)
b)
9.
a) Mg2+
b)
11.
a) Fe2+
Lewis Dot Structure Bond Calculator Equation
b)